- The COVID-19 Vaccine Safety Research Center: a cornerstone for strengthening safety evidence for COVID-19 vaccination in the Republic of Korea
-
Na-Young Jeong, Hyesook Park, Sanghoon Oh, Seung Eun Jung, Dong-Hyun Kim, Hyoung-Shik Shin, Hee Chul Han, Jong-Koo Lee, Jun Hee Woo, Jaehun Jung, Joongyub Lee, Ju-Young Shin, Sun-Young Jung, Byung-Joo Park, Nam-Kyong Choi
-
Osong Public Health Res Perspect. 2024;15(2):97-106. Published online April 4, 2024
-
DOI: https://doi.org/10.24171/j.phrp.2023.0343
-
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
 - The COVID-19 Vaccine Safety Research Committee (CoVaSC) was established in November 2021 to address the growing need for independent, in-depth scientific evidence on adverse events (AEs) following coronavirus disease 2019 (COVID-19) vaccination. This initiative was requested by the Korea Disease Control and Prevention Agency and led by the National Academy of Medicine of Korea. In September 2022, the COVID-19 Vaccine Safety Research Center was established, strengthening CoVaSC’s initiatives. The center has conducted various studies on the safety of COVID-19 vaccines. During CoVaSC’s second research year, from September 29, 2022 to July 19, 2023, the center was restructured into 4 departments: Epidemiological Research, Clinical Research, Communication & Education, and International Cooperation & Policy Research. Its main activities include (1) managing CoVaSC and the COVID-19 Vaccine Safety Research Center, (2) surveying domestic and international trends in AE causality investigation, (3) assessing AEs following COVID-19 vaccination, (4) fostering international collaboration and policy research, and (5) organizing regular fora and training sessions for the public and clinicians. Causality assessments have been conducted for 27 diseases, and independent research has been conducted after organizing ad hoc committees comprising both epidemiologists and clinical experts on each AE of interest. The research process included protocol development, data analysis, interpretation of results, and causality assessment. These research outcomes have been shared transparently with the public and healthcare experts through various fora. The COVID-19 Vaccine Safety Research Center plans to continue strengthening and expanding its research activities to provide reliable, high-quality safety information to the public.
- Developing a national surveillance system for stroke and acute myocardial infarction using claims data in the Republic of Korea: a retrospective study
-
Tae Jung Kim, Hak Seung Lee, Seong-Eun Kim, Jinju Park, Jun Yup Kim, Jiyoon Lee, Ji Eun Song, Jin-Hyuk Hong, Joongyub Lee, Joong-Hwa Chung, Hyeon Chang Kim, Dong-Ho Shin, Hae-Young Lee, Bum Joon Kim, Woo-Keun Seo, Jong-Moo Park, Soo Joo Lee, Keun-Hwa Jung, Sun U. Kwon, Yun-Chul Hong, Hyo-Soo Kim, Hyun-Jae Kang, Juneyoung Lee, Hee-Joon Bae
-
Osong Public Health Res Perspect. 2024;15(1):18-32. Published online January 31, 2024
-
DOI: https://doi.org/10.24171/j.phrp.2023.0248
-
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
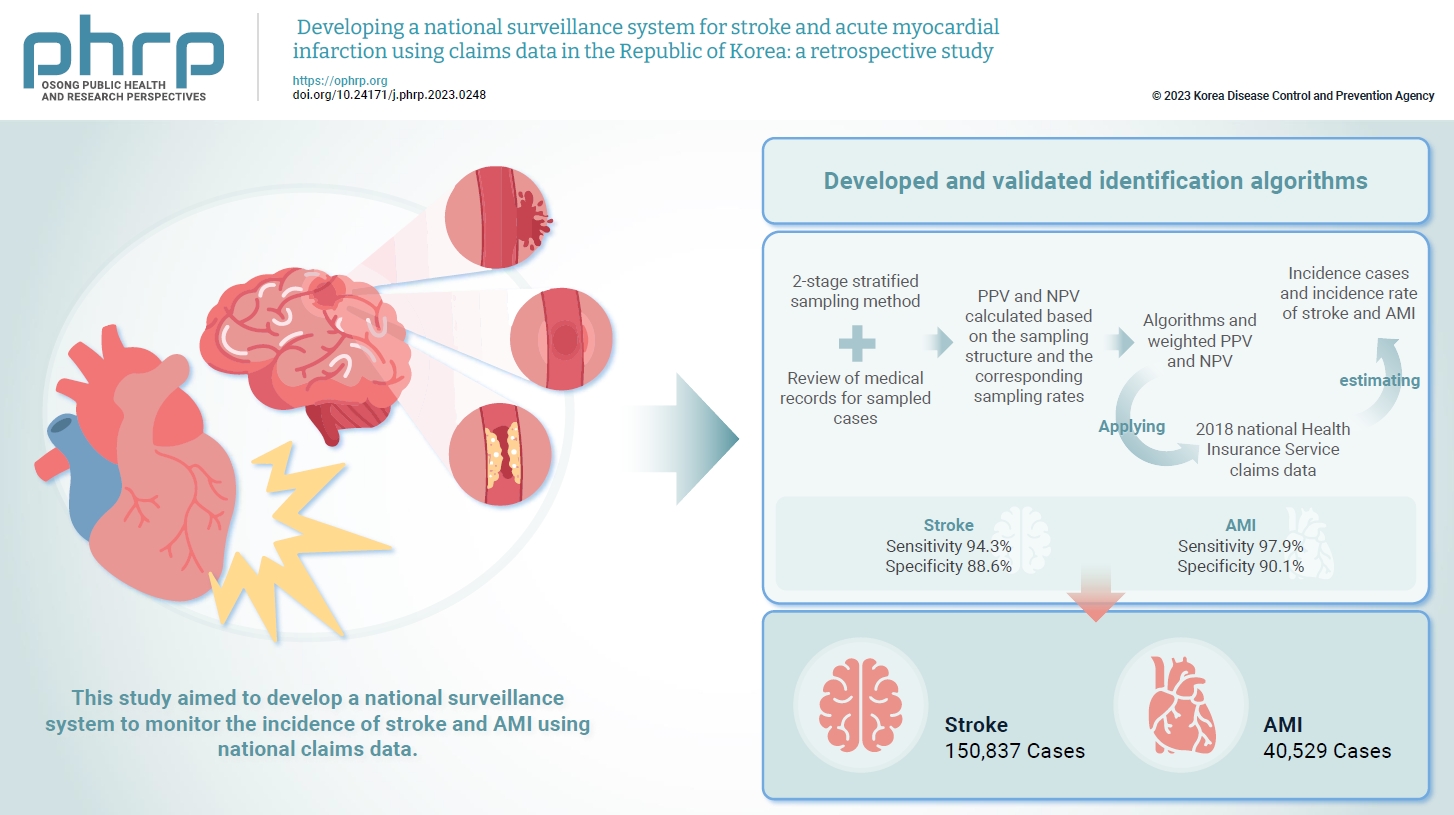 - Objectives
Limited information is available concerning the epidemiology of stroke and acute myocardial infarction (AMI) in the Republic of Korea. This study aimed to develop a national surveillance system to monitor the incidence of stroke and AMI using national claims data. Methods: We developed and validated identification algorithms for stroke and AMI using claims data. This validation involved a 2-stage stratified sampling method with a review of medical records for sampled cases. The weighted positive predictive value (PPV) and negative predictive value (NPV) were calculated based on the sampling structure and the corresponding sampling rates. Incident cases and the incidence rates of stroke and AMI in the Republic of Korea were estimated by applying the algorithms and weighted PPV and NPV to the 2018 National Health Insurance Service claims data. Results: In total, 2,200 cases (1,086 stroke cases and 1,114 AMI cases) were sampled from the 2018 claims database. The sensitivity and specificity of the algorithms were 94.3% and 88.6% for stroke and 97.9% and 90.1% for AMI, respectively. The estimated number of cases, including recurrent events, was 150,837 for stroke and 40,529 for AMI in 2018. The age- and sex-standardized incidence rate for stroke and AMI was 180.2 and 46.1 cases per 100,000 person-years, respectively, in 2018. Conclusion: This study demonstrates the feasibility of developing a national surveillance system based on claims data and identification algorithms for stroke and AMI to monitor their incidence rates.
|